
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 67.7 g of sulfuric acid is mixed with 83. g of sodium hydroxide. Calculate the minimum mass of sulfuric acid that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
Do you know the correct answer?
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and l...
Questions in other subjects:


Mathematics, 24.08.2019 19:50

History, 24.08.2019 19:50

History, 24.08.2019 20:00



Biology, 24.08.2019 20:00

History, 24.08.2019 20:00

Biology, 24.08.2019 20:00


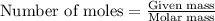
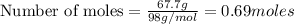
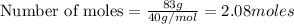
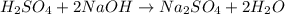
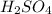 require 2 moles of
require 2 moles of 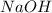
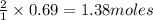 of
of 




