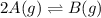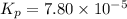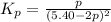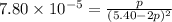Consider the reaction.
2A(g)−⇀↽−B(g) Kp=7.80×10−5
at 500 K If a sample of A(g) at 5.40 a...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1



Chemistry, 23.06.2019 02:30, ggpro4life3000
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2
Do you know the correct answer?
Questions in other subjects:






Mathematics, 03.07.2020 20:01