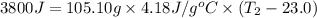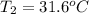Chemistry, 11.03.2020 22:58, spotty1023
In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used. The initial temperature of the calorimeter is 23.0 ∘C. If 5.10 g of CaCl2 is added to the calorimeter, what will be the final temperature of the solution in the calorimeter? The heat of solution ΔHsoln of CaCl2 is −82.8 kJ/mol .

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, 4tazaouiamine1r
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2



Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
Do you know the correct answer?
In the following experiment, a coffee-cup calorimeter containing 100 mL of H2O is used. The initial...
Questions in other subjects:

English, 12.10.2019 12:30



Mathematics, 12.10.2019 12:30


Geography, 12.10.2019 12:30

Mathematics, 12.10.2019 12:30


Computers and Technology, 12.10.2019 12:30

Mathematics, 12.10.2019 12:30


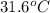
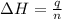
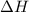 = enthalpy change = 82.8 kJ/mol
= enthalpy change = 82.8 kJ/mol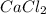 = 5.10 g
= 5.10 g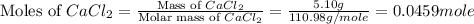
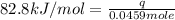
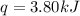
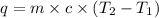
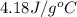
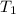 = initial temperature =
= initial temperature = 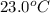
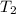 = final temperature = ?
= final temperature = ?