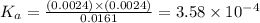Chemistry, 11.03.2020 22:13, msjsnell29
A weak monoprotic acid has molar mass 180 g/mol. When 1.00 g of this acid is dissolved in enough water to obtain a 300 mL solution, the pH of the resulting solution is found to be 2.62. What is the value of Ka for this acid

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
Do you know the correct answer?
A weak monoprotic acid has molar mass 180 g/mol. When 1.00 g of this acid is dissolved in enough wat...
Questions in other subjects:




Social Studies, 14.06.2021 14:00


English, 14.06.2021 14:00

Spanish, 14.06.2021 14:00

Chemistry, 14.06.2021 14:00

Social Studies, 14.06.2021 14:00


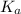 for the given acid is
for the given acid is 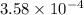
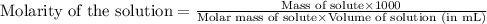
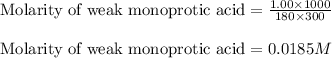
![pH=-\log[H^+]](/tpl/images/0543/4513/cf945.png)
![2.62=-\log[H^+]](/tpl/images/0543/4513/2e60f.png)
![[H^+]=10^{-2.62}=2.40\times 10^{-3}M=0.0024M](/tpl/images/0543/4513/dcf01.png)
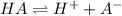
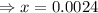
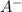 = x = 0.0024 M
= x = 0.0024 M![K_a=\frac{[H^+][A^-]}{[HA]}](/tpl/images/0543/4513/66f51.png)