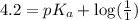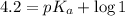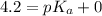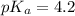Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 19:40, powberier6979
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
Do you know the correct answer?
An unknown acid is titrated with a strong base. At the half-equivalence point (i. e., at the volume...
Questions in other subjects:

Mathematics, 21.11.2020 08:00

Business, 21.11.2020 08:00

Chemistry, 21.11.2020 08:00


Arts, 21.11.2020 08:00



English, 21.11.2020 08:00

English, 21.11.2020 08:00


![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0543/2312/e961a.png)