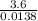Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3


Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
Do you know the correct answer?
Calculate the numerical Kc value for the following reaction if the equilibrium mixture contains 0.51...
Questions in other subjects:


Social Studies, 15.12.2020 06:40


Biology, 15.12.2020 06:40



Arts, 15.12.2020 06:40

Mathematics, 15.12.2020 06:40

Social Studies, 15.12.2020 06:40


![\frac{[CH_{4}][H_{2}O]}{[CO][H_{2}]^{3}}](/tpl/images/0543/2078/e3248.png)
![\frac{[1.8][2]}{[0.51][0.3]^{3}}](/tpl/images/0543/2078/1746f.png)