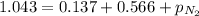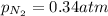Chemistry, 11.03.2020 18:22, makrosebud7821
A mixture of He, NE, and N2 gases has a pressure of 1.043 atm. If the pressures of He and Ne are 0.137 atm and 0.566 atm, respectively, what is the partial pressure of N2 in the mixture?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2

Chemistry, 23.06.2019 08:00, mshields1994
Amechanical wave that transports a lot of energy will have a
Answers: 2
Do you know the correct answer?
A mixture of He, NE, and N2 gases has a pressure of 1.043 atm. If the pressures of He and Ne are 0.1...
Questions in other subjects:

Arts, 24.06.2019 01:20

Mathematics, 24.06.2019 01:20



Mathematics, 24.06.2019 01:20



Biology, 24.06.2019 01:20


History, 24.06.2019 01:20


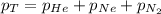
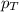 = total pressure of gas = 1.043 atm
= total pressure of gas = 1.043 atm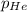 = partial pressure of helium gas = 0.137 atm
= partial pressure of helium gas = 0.137 atm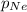 = partial pressure of neon gas = 0.566 atm
= partial pressure of neon gas = 0.566 atm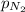 = partial pressure of nitrogen gas = ?
= partial pressure of nitrogen gas = ?