
Chemistry, 11.03.2020 17:49, tylerkitchen44
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below?
2c2H6(g)+7O2(g)--->4CO2(g)+6H2O( g)
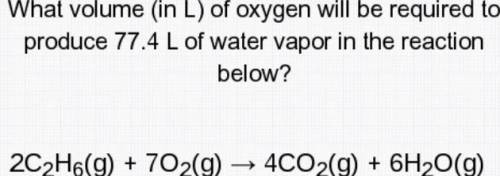

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2


Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
Do you know the correct answer?
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below...
Questions in other subjects:

Mathematics, 17.07.2019 13:30



Mathematics, 17.07.2019 13:30

Biology, 17.07.2019 13:30



Mathematics, 17.07.2019 13:30







