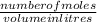A chemist prepares a solution of silver(I) nitrate AgNO3 by measuring out ×4.7102μmol of silver(I) nitrate into a 450.mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in /mmolL of the chemist's silver(I) nitrate solution. Be sure your answer has the correc

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3
Do you know the correct answer?
A chemist prepares a solution of silver(I) nitrate AgNO3 by measuring out ×4.7102μmol of silver(I) n...
Questions in other subjects:


Social Studies, 22.12.2021 06:50




SAT, 22.12.2021 06:50



Social Studies, 22.12.2021 06:50