PLEASE HELP!
1. Determine whether or not the equation below is balanced. If it isn’t balanced,...

PLEASE HELP!
1. Determine whether or not the equation below is balanced. If it isn’t balanced, write the balanced form. Also, identify the reactant(s) and product(s) in this equation. Finally, label this as one of the five types of reactions: combination, decomposition, substitution, double replacement, or reversible.
Zn+HCl→ZnCl2+H2 Zn+ HCl→ ZnCl2+ H2
2. Determine whether or not the equation below is balanced. If it isn’t balanced, write the balanced form. Also, identify the reactant(s) and product(s) in this equation. Finally, label this as one of the five types of reactions: combination, decomposition, substitution, double replacement, or reversible.
S8+24F2→8SF6 S8+ 24F2→ 8SF6
3. Calculate the molecular mass of ferric oxide (Fe3O4).
4. Determine the percentage composition of chlorine in CaCl2.
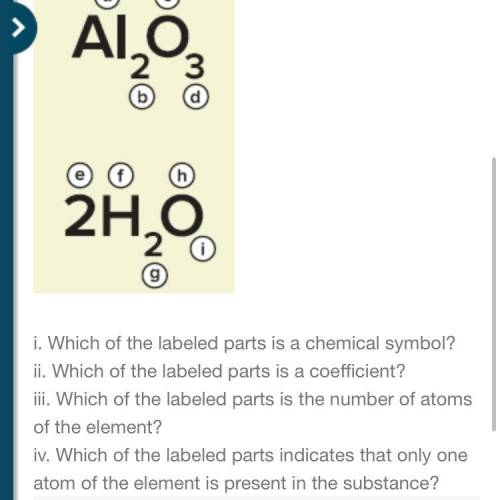
5. Identify the labeled parts in the figure.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1


Chemistry, 23.06.2019 15:00, swelch2010
The atoms in a have a definite volume, but move quickly enough to overcome the forces of attraction between them. a. solid b. liquid c. gas
Answers: 2
Do you know the correct answer?
Questions in other subjects:















