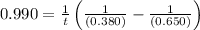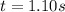Chemistry, 11.03.2020 06:13, dianacastro8298
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 300 ∘C. A ⟶ products A⟶products How long, in seconds, would it take for the concentration of A A to decrease from 0.650 M 0.650 M to 0.380 M?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 00:50, alainacorkell6472
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:20, hflores0001
How can parts of a solution be separated by chromatography?
Answers: 1
Do you know the correct answer?
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 30...
Questions in other subjects:

Computers and Technology, 23.05.2020 03:02

Biology, 23.05.2020 03:02


English, 23.05.2020 03:02



Mathematics, 23.05.2020 03:02

Mathematics, 23.05.2020 03:02


Mathematics, 23.05.2020 03:02


![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0542/6727/5ea71.png)
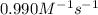
![[A]_o](/tpl/images/0542/6727/9caf5.png) = Initial concentration = 0.650 M
= Initial concentration = 0.650 M