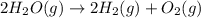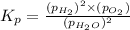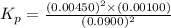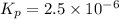Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Do you know the correct answer?
The elementary reaction 2H20(g)<--->2H2(g)+O2(g) proceeds at a certain temperature until the p...
Questions in other subjects:

History, 21.05.2021 16:20


Social Studies, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20




Mathematics, 21.05.2021 16:20

Mathematics, 21.05.2021 16:20


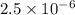
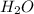 = 0.0900 atm
= 0.0900 atm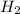 = 0.00450 atm
= 0.00450 atm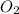 = 0.00100 atm
= 0.00100 atm