
Chemistry, 11.03.2020 03:59, emmaraeschool
A 101 g piece of aluminum (aluminum = 0.900 J/g oC) is heated to 100.1 oC and added to 48.9 g of water at an initial temperature of 16.5oC. what will the final temperature of the mixture be

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1


Chemistry, 23.06.2019 07:00, bree6754
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
Do you know the correct answer?
A 101 g piece of aluminum (aluminum = 0.900 J/g oC) is heated to 100.1 oC and added to 48.9 g of wat...
Questions in other subjects:


History, 29.01.2021 20:10

Advanced Placement (AP), 29.01.2021 20:10

History, 29.01.2021 20:10

History, 29.01.2021 20:10

Mathematics, 29.01.2021 20:10


Engineering, 29.01.2021 20:10

Chemistry, 29.01.2021 20:10


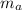 = 101 gm
= 101 gm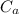 = 0.9
= 0.9 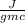
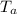 = 100.1 °C
= 100.1 °C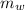 = 48.9 gm
= 48.9 gm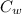 = 4.2
= 4.2 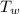 = 16.5 °C
= 16.5 °C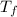 ) =
) = 



