
The following equation shows the equilibrium in an aqueous solution of ammonia: NH3(aq)+H2O(l)⇌NH4+(aq)+OH−(aq)NH3( aq)+H2O(l)⇌NH4+(aq)+OH−(aq) Which of the following represents a conjugate acid-base pair?a) NH3 and H2O b) NH4+ and OH− c) H2O and OH− d) NH3 and OH−

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 00:30, vane6176
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
Do you know the correct answer?
The following equation shows the equilibrium in an aqueous solution of ammonia: NH3(aq)+H2O(l)⇌NH4+(...
Questions in other subjects:


Physics, 09.06.2020 06:57



Mathematics, 09.06.2020 06:57






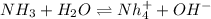
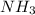 and the conjugate acid of the base is
and the conjugate acid of the base is 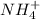 .
.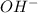 and the conjugate acid of the base is
and the conjugate acid of the base is 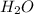 .
.



