
Chemistry, 11.03.2020 02:25, unicornpoop54
A certain chemical reaction releases 480. kJ of heat energy per mole of reactant consumed. Suppose some moles of the reactant are put into a calorimeter (a device for measuring heat flow). It takes 3.85 J of heat energy to raise the temperature of this calorimeter by 1 °C. Now the reaction is run until all the reactant is gone, and the temperature of the calorimeter is found to rise by 14.6 °C. How would you calculate the number of moles of reactant that were consumed? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. moles consumed-

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2


Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
Do you know the correct answer?
A certain chemical reaction releases 480. kJ of heat energy per mole of reactant consumed. Suppose s...
Questions in other subjects:

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

English, 10.09.2020 14:01


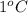 . So here, specific heat is given as 3.85 J. And, the change in temperature is
. So here, specific heat is given as 3.85 J. And, the change in temperature is 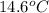 .
.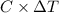
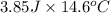
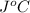
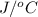 of heat is calculated as follows.
of heat is calculated as follows.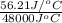
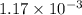 moles
moles



