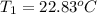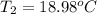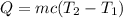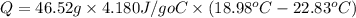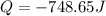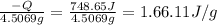Chemistry, 11.03.2020 02:11, zacharycheyne
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperature change. The specific heat of water is 4.180 Joules per g per ºC. In the calculation of the heat of solution, ignore the contribution to specific heat and mass due to the salt. Assume that these contributions are negligible. The data collected are as follows:
Grams of water in the calorimeter 46.52
Grams of salt 4.5069
Initial temperature of water 22.83 ºC
Final Temperature 18.98 ºC
Calculate the following Heat of the solution of salt.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1



Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
Do you know the correct answer?
The heat of solution is found by adding a salt to water in a calorimeter and measuring the temperatu...
Questions in other subjects:


Mathematics, 19.12.2019 14:31





English, 19.12.2019 14:31


World Languages, 19.12.2019 14:31