
Chemistry, 10.03.2020 22:29, chickenwing32
For the reaction 2 HCl + Na2CO3 → 2 NaCl + H2O + CO2 8.0 moles of CO2 is collected at STP. What is the volume of CO2? 1) 57.6 L 2) 22.4 L 3) 0.0250 L 4) 2.80 L 5) 179 L 6) 0.357 L

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2


Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
Do you know the correct answer?
For the reaction 2 HCl + Na2CO3 → 2 NaCl + H2O + CO2 8.0 moles of CO2 is collected at STP. What is t...
Questions in other subjects:





Spanish, 17.02.2022 16:50






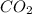 = 8.0 mole
= 8.0 mole
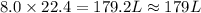 volume of
volume of 




