
Chemistry, 10.03.2020 22:15, taniyahbenyamin2
Data for the decomposition of hydrogen peroxide at some set temperature T is provided below. The rate law depends only on the concentration of H2O2. (These same data will be used for questions 6, 7 and 8.) 2 H2O2 ---> 2 H2O O2 t (seconds) 0 60 120 180 240 360 420 600 [H2O2] (M) 0.882 0.697 0.566 0.458 0.372 0.236 0.188 0.094 What is the value of the rate constant, k

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
Do you know the correct answer?
Data for the decomposition of hydrogen peroxide at some set temperature T is provided below. The rat...
Questions in other subjects:

Mathematics, 13.05.2021 16:50

Mathematics, 13.05.2021 16:50


Mathematics, 13.05.2021 16:50

English, 13.05.2021 16:50



Mathematics, 13.05.2021 16:50


Mathematics, 13.05.2021 16:50


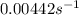 is the value of the rate constant.
is the value of the rate constant.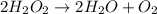
![R=k[H_2O_2]^x](/tpl/images/0541/5739/94d73.png)
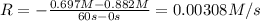
![0.00308 M/s=k[0.697 M]^x](/tpl/images/0541/5739/443b7.png) ..[1]
..[1]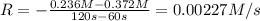
![0.00218 M/s=k[0.236 M]^x](/tpl/images/0541/5739/bccb9.png) ..[2]
..[2]![\frac{0.00308 M/s}{0.00227 M/s}=\frac{k[0.697 M]^x}{k[0.236M]^x}](/tpl/images/0541/5739/1e2cd.png)
![R=k[H_2O_2]^1](/tpl/images/0541/5739/48c78.png)
![0.00308 M/s=k[0.697 M]^1](/tpl/images/0541/5739/15c46.png)
![k=\frac{0.00308 M/s}{[0.697 M]^1}=0.00442 s^{-1}](/tpl/images/0541/5739/22700.png)




