
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. H+ + H2O2 ? H3O2+ (rapid equilibrium) H3O2+ + Br- ? HOBr + H2O (slow) HOBr+H+ +Br- ?Br2 +H2O(fast) Which rate law is consistent with this mechanism?
a. k[Br-][H+]-1[H2O2]-1
b. k[H+][H2O2][Br-]
c. k[H+][H2O2]
d. k[HOBr][H+][Br-]

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1


Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Do you know the correct answer?
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous a...
Questions in other subjects:



Mathematics, 09.02.2021 18:40

English, 09.02.2021 18:40




Mathematics, 09.02.2021 18:40



![\text{Rate}=k'[H+][H_2O_2][Br^-]](/tpl/images/0541/5354/e35f3.png)
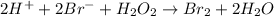
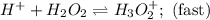
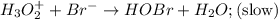
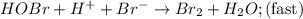
![\text{Rate}=k[H_3O_2^+][Br^-]](/tpl/images/0541/5354/37d2f.png) ......(1)
......(1)![[H_3O_2^+]](/tpl/images/0541/5354/85c88.png) is not appearing as a reactant in the overall reaction. So, we apply steady state approximation in it.
is not appearing as a reactant in the overall reaction. So, we apply steady state approximation in it.![K=\frac{[H_3O_2^+]}{[H^+][H_2O_2]}](/tpl/images/0541/5354/4cd3a.png)
![[H_3O_2^+]=K[H^+][H_2O_2]](/tpl/images/0541/5354/15cd9.png)
![\text{Rate}=k.K[H^+][H_2O_2][Br^-]\\\\\text{Rate}=k'[H+][H_2O_2][Br^-]](/tpl/images/0541/5354/f918c.png)




