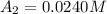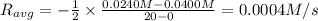Chemistry, 10.03.2020 22:12, chloesmolinski0909
(a) The rate of the reaction in terms of the "disappearance of reactant" includes the change in the concentration of the
reactant, the time interval, and the coefficient of the reactant.
Consider the following reaction:
2A+3B > 3C+2D
The concentrations of reactant A at three different time intervals are given. Use the following data to determine the average rate of reaction in terms of the disappearance of reactant A between time = 0 s and time = 20 s .
Time (s) 0 20 40
[A](M) 0.0400 0.0240 0.0180
Express your answer in molar concentration per second to three significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
Do you know the correct answer?
(a) The rate of the reaction in terms of the "disappearance of reactant" includes the change in the...
Questions in other subjects:

Mathematics, 28.04.2021 04:20



Mathematics, 28.04.2021 04:20

Mathematics, 28.04.2021 04:20

Mathematics, 28.04.2021 04:20




Medicine, 28.04.2021 04:20


![R_{avg}=-\frac{[A]_2-[A]_1}{t_2-t_1}](/tpl/images/0541/5709/9f5c2.png)
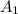 = initial concentration of reactant at
= initial concentration of reactant at 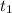 .
.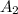 = Final concentration of reactant at
= Final concentration of reactant at 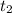 .
.![R_{avg}=-\frac{1}{2}\frac{[A]_2-[A]_1}{t_2-t_1}](/tpl/images/0541/5709/30159.png)
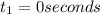 ) =
) = 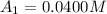
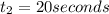 ) =
) =