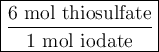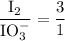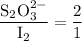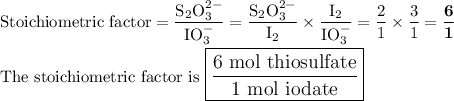Part III. The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(aq) 5 I-(aq) 6 H (aq) --> 3 I2(aq) 3 H2O(l) followed by reaction of the I2: I2(aq) 2 S2O32- --> 2 I-(aq) S4O62-(aq). What is the stoichiometric factor, that is the number of moles of Na2S2O3 reacting with one mole of KIO3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
Do you know the correct answer?
Part III. The two reactions involved in quantitatively determining the amount of iodate in solution...
Questions in other subjects:

Mathematics, 10.02.2021 03:20

Mathematics, 10.02.2021 03:20

Mathematics, 10.02.2021 03:20




Physics, 10.02.2021 03:20


Chemistry, 10.02.2021 03:20

Mathematics, 10.02.2021 03:20