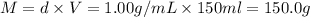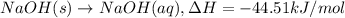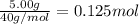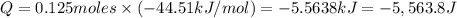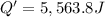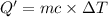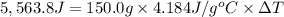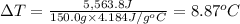Chemistry, 10.03.2020 19:01, gooberthebear8955
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in temperature of a coffee cup calorimeter containing 150 ml of water when 5.00 g NaOH (40.00 g/mol) is added to the container. You may assume that the solution has the same specific heat and density as water.
NaOH(s) → NaOH(aq) ΔH =-44.51 KJ
a. +8.87°C
b. +2.70 C
c. 2.70°C
d. 8.87°C

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, YoEsMyles3115
0.2348 grams of pbcl2 used to form 44.0 ml of solution.
Answers: 1

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
Do you know the correct answer?
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in te...
Questions in other subjects:


Engineering, 13.09.2019 23:20






Engineering, 13.09.2019 23:20