

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2
Do you know the correct answer?
Reaction rate is expressed in terms of changes in the concentration of reactants and products. Write...
Questions in other subjects:

Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

History, 16.10.2020 05:01

Chemistry, 16.10.2020 05:01


English, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01


Biology, 16.10.2020 05:01


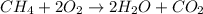
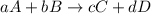
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0541/3019/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0541/3019/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0541/3019/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/d4b94.png)
![Rate=-\frac{d[CH_4]}{dt}=-\frac{1}{2}\frac{d[O_2]}{dt}=+\frac{1}{2}\frac{d[H_2O]}{dt}=+\frac{d[CO_2]}{dt}](/tpl/images/0541/3019/dfd60.png)




