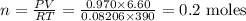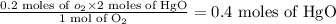Chemistry, 10.03.2020 18:38, sahaitong1844
An unknown amount of mercury (II) oxide was decomposed in the lab. Mercury metal
was formed and 6.60 L of oxygen gas was released at a pressure of 0.970 atm and
390.0 K. What was the initial weight of mercury oxide in the sample? (1 point)
1) 48.91 grams
2) 64.32 grams
3) 78.32 grams
4) 86.6 grams

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2


Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1
Do you know the correct answer?
An unknown amount of mercury (II) oxide was decomposed in the lab. Mercury metal
was formed an...
was formed an...
Questions in other subjects:



Mathematics, 05.01.2021 18:00

Arts, 05.01.2021 18:00

History, 05.01.2021 18:00

Mathematics, 05.01.2021 18:00

English, 05.01.2021 18:00

French, 05.01.2021 18:00

Mathematics, 05.01.2021 18:00