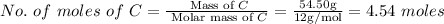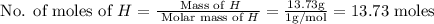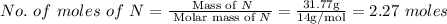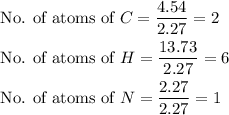The rotten smell of a decaying animal carcass is partially due to a nitrogen-containing compound called putrescine. Elemental analysis of putrescine indicates that it consists of:
C
,
54.50
%
,
H
,
13.73
%
, and
N
,
31.77
%
. Calculate the empirical formula of putrescine.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 04:20, vliu732
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
Do you know the correct answer?
The rotten smell of a decaying animal carcass is partially due to a nitrogen-containing compound cal...
Questions in other subjects:


Physics, 25.08.2019 19:30

History, 25.08.2019 19:30

Mathematics, 25.08.2019 19:30


Chemistry, 25.08.2019 19:30

Mathematics, 25.08.2019 19:30


Mathematics, 25.08.2019 19:30


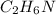 .
.