
Chemistry, 10.03.2020 17:34, stormmesa1
An element P combines with another element Q in the weight ratio 1: 5. If 15 g of P is made to react with 62g of Q to a compound. What will be the total weight of the new compound formed?
(A) 60 g
(B) 77 g
(C) 74.4 g
(D) 74 g
(E) 66 g

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1


Do you know the correct answer?
An element P combines with another element Q in the weight ratio 1: 5. If 15 g of P is made to react...
Questions in other subjects:


French, 23.05.2021 07:40







Biology, 23.05.2021 07:40

History, 23.05.2021 07:40


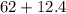 grams which will give us with 74.4 grams of compound.
grams which will give us with 74.4 grams of compound. 



