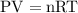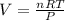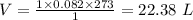3. The molar volume of a gas at STP occupies
0 1 kilopascal
O ooc
12 grams
2...

Chemistry, 10.03.2020 17:15, bhuminaik532
3. The molar volume of a gas at STP occupies
0 1 kilopascal
O ooc
12 grams
22.4 L

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 02:30, roseemariehunter12
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 10:30, EstherAbuwaah
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh. the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Mathematics, 31.08.2021 08:20


Mathematics, 31.08.2021 08:20

Mathematics, 31.08.2021 08:20


Mathematics, 31.08.2021 08:20

Social Studies, 31.08.2021 08:20

Mathematics, 31.08.2021 08:20

Business, 31.08.2021 08:20

Mathematics, 31.08.2021 08:20