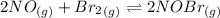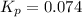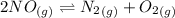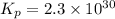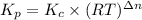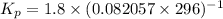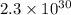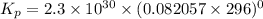Chemistry, 10.03.2020 09:56, mattmaddox86
Calculate the equilibrium constant Kp for this reaction, given the following information (at 296 K ): 2NO(g)+Br2(g)⇌2NOBr(g)Kc=1.8 2NO(g)⇌N2(g)+O2(g)Kc=2.3×1030

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 08:00, mshields1994
Amechanical wave that transports a lot of energy will have a
Answers: 2
Do you know the correct answer?
Calculate the equilibrium constant Kp for this reaction, given the following information (at 296 K )...
Questions in other subjects:

Computers and Technology, 26.02.2021 20:50






Mathematics, 26.02.2021 20:50

Computers and Technology, 26.02.2021 20:50


Mathematics, 26.02.2021 20:50