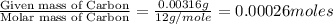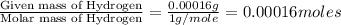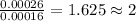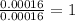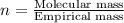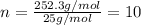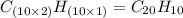Chemistry, 10.03.2020 09:06, auviannadority13
One of the components of natural crude oil and coal deposits is benzo[a]pyrene, a compound with a molecular mass of about 252.3 amu, containing only carbon and hydrogen. A 3.320 mg sample of benzo[a]pyrene burns to give 11.58 mg of CO2. Determine its empirical and molecular formulas. (Omit states-of-matter from your answer.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 19:40, trodgers0202
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests. which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
Do you know the correct answer?
One of the components of natural crude oil and coal deposits is benzo[a]pyrene, a compound with a mo...
Questions in other subjects:


Geography, 08.03.2021 18:30




Mathematics, 08.03.2021 18:30

Mathematics, 08.03.2021 18:30




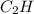 and
and 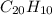
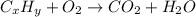
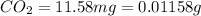 (Conversion factor: 1 g = 1000 mg)
(Conversion factor: 1 g = 1000 mg)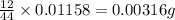 of carbon will be contained.
of carbon will be contained.