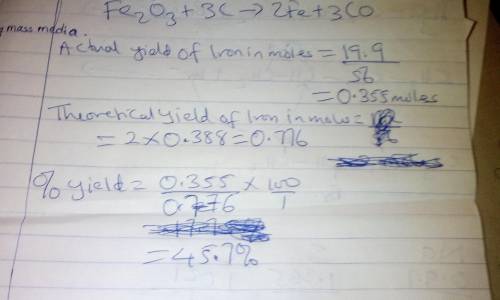Chemistry, 10.03.2020 08:05, quanwalker651370
Combining 0.338 mol Fe 2 O 3 with excess carbon produced 19.9 g Fe . Fe 2 O 3 + 3 C ⟶ 2 Fe + 3 CO What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? theoretical yield: mol What is the percent yield? percent yield:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
Do you know the correct answer?
Combining 0.338 mol Fe 2 O 3 with excess carbon produced 19.9 g Fe . Fe 2 O 3 + 3 C ⟶ 2 Fe + 3 CO Wh...
Questions in other subjects:







English, 23.03.2021 23:10


History, 23.03.2021 23:10