
Chemistry, 10.03.2020 08:06, loganhowington26
Consider the reaction. 2 Al ( s ) + Fe 2 O 3 ( s ) heat −−→ Al 2 O 3 ( s ) + 2 Fe ( l ) If 17.3 kg Al reacts with an excess of Fe 2 O 3 , how many kilograms of Al 2 O 3 will be produced?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


Chemistry, 22.06.2019 22:30, darkshaders11
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
Do you know the correct answer?
Consider the reaction. 2 Al ( s ) + Fe 2 O 3 ( s ) heat −−→ Al 2 O 3 ( s ) + 2 Fe ( l ) If 17.3 kg A...
Questions in other subjects:


Computers and Technology, 13.10.2020 08:01




Law, 13.10.2020 08:01

English, 13.10.2020 08:01


Chemistry, 13.10.2020 08:01

English, 13.10.2020 08:01


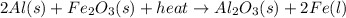
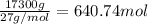
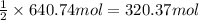 of aluminum oxide
of aluminum oxide



