
Chemistry, 10.03.2020 08:21, lovebunny33921
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature with initial concentrations of [CO]=0.27M[CO]=0.27M and [H2]=0.49M[H2]=0.49M. At equilibrium, the concentration of CH3OHCH3OH is 0.11 MM. Find the equilibrium constant at this temperature.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, dustinquiz255
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
Do you know the correct answer?
CO(g)+2H2(g)⇌CH3OH(g)CO(g)+2H2(g)⇌C H3OH(g) This reaction is carried out at a different temperature...
Questions in other subjects:

Mathematics, 04.06.2021 17:50


Social Studies, 04.06.2021 17:50

Mathematics, 04.06.2021 17:50

Social Studies, 04.06.2021 17:50

History, 04.06.2021 17:50


Social Studies, 04.06.2021 17:50

History, 04.06.2021 17:50


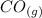 +
+ 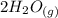 ⇄
⇄ 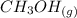
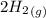 ⇄
⇄ ![K_C = \frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0540/6074/3667b.png)
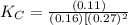
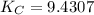
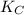 = 9.4
= 9.4




