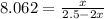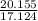Chemistry, 10.03.2020 08:14, depinedainstcom
At 25°C, the equilibrium constant Kc for the reaction 2A(aq) ↔ B(aq) + C(aq) is 65. If 2.50 mol of A is added to enough water to prepare 1.00 L of solution, what will the equilibrium concentration of A be?

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
Do you know the correct answer?
At 25°C, the equilibrium constant Kc for the reaction 2A(aq) ↔ B(aq) + C(aq) is 65. If 2.50 mol of A...
Questions in other subjects:


History, 20.11.2020 23:00

English, 20.11.2020 23:00



Mathematics, 20.11.2020 23:00




Mathematics, 20.11.2020 23:00


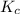 = 65
= 65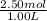
![K_c =\frac {[B][C]}{[A]^2}](/tpl/images/0540/5702/5350b.png)
![65 = \frac{[x][x]}{[2.5-2x]^2}](/tpl/images/0540/5702/bd4f1.png)
![65 = \frac{[x]^2}{[2.5-2x]^2}](/tpl/images/0540/5702/0dd40.png)
![65 = (\frac{[x]}{[2.5-2x]})^2](/tpl/images/0540/5702/0c0f2.png)
![\sqrt 65 = \sqrt {(\frac{[x]}{[2.5-2x]})^2}](/tpl/images/0540/5702/bb4a3.png)