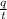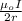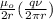Chemistry, 10.03.2020 07:55, Queenhagar
In Niels Bohr's 1913 model of the hydrogen atom, an electron circles the proton at a distance of 5.29 10-11 m with a speed of 2.19 106 m/s. Compute the magnitude of the magnetic field this motion produces at the location of the proton.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Do you know the correct answer?
In Niels Bohr's 1913 model of the hydrogen atom, an electron circles the proton at a distance of 5.2...
Questions in other subjects:

Mathematics, 21.09.2021 03:10


Mathematics, 21.09.2021 03:10

Mathematics, 21.09.2021 03:10

Mathematics, 21.09.2021 03:10



English, 21.09.2021 03:10

English, 21.09.2021 03:10

English, 21.09.2021 03:10


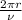
 = speed of electron
= speed of electron