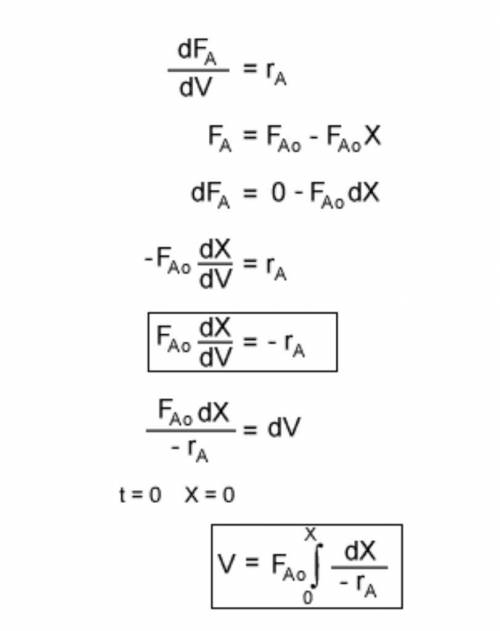
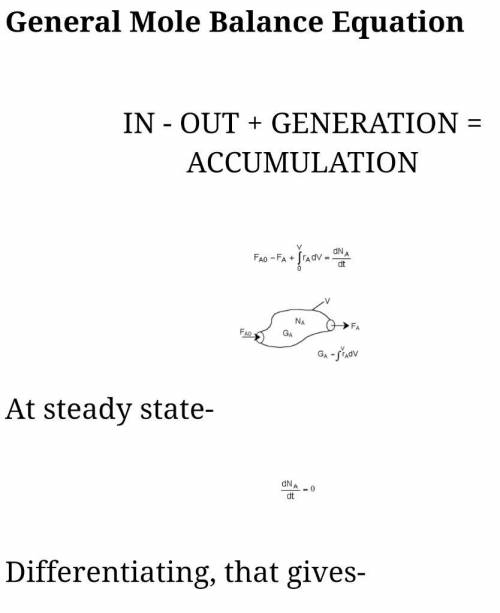
The irreversible decomposition of the di-tert-butyl peroxide is to carried out in an isothermal PFR in which there is no pressure drop. Symbolically, this reaction can be written A > B 2C. The feed consists of di-tert-butyl peroxide and inert nitrogen. The reactor volume is 200 dm3, and the entering volumetric flow rate is maintained constant at 10 dm3/min. The reaction rate constant k for this first-order reaction is 0.08 min-1 which is based on reactant A. For this reactor system, write;(a) the material balance equation(b) the rate law equation

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1


Do you know the correct answer?
The irreversible decomposition of the di-tert-butyl peroxide is to carried out in an isothermal PFR...
Questions in other subjects:

Mathematics, 26.10.2020 18:40

Mathematics, 26.10.2020 18:40

Physics, 26.10.2020 18:40



History, 26.10.2020 18:40

English, 26.10.2020 18:40


Mathematics, 26.10.2020 18:40