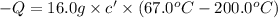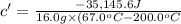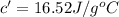Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:50, lildestinyquintana
Ase your answer to this question on the information below. hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant. the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen. chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product. nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1


Chemistry, 23.06.2019 12:40, valleriieZ7002
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2
Do you know the correct answer?
16.0 g copper pan at 200.0∘C is plunged into 200.0 mL of water at 25.0∘C causing the water to rise t...
Questions in other subjects:








Mathematics, 27.06.2020 18:01


Mathematics, 27.06.2020 19:01


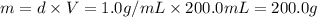
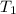 = 25.0°C
= 25.0°C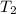 = 67.0°C
= 67.0°C

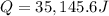
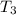 = 200.0 °C
= 200.0 °C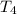 = 67.0°C
= 67.0°C