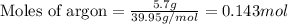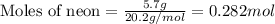Chemistry, 10.03.2020 07:22, lololol270
A gas mixture is made by combining 5.7 g each of Ar , Ne , and an unknown diatomic gas. At STP, the mixture occupies a volume of 12.88 L. What is the molar mass of the unknown gas?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 04:00, Bassoonist
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
Do you know the correct answer?
A gas mixture is made by combining 5.7 g each of Ar , Ne , and an unknown diatomic gas. At STP, the...
Questions in other subjects:

Mathematics, 09.04.2021 07:20

Mathematics, 09.04.2021 07:20


Mathematics, 09.04.2021 07:20

English, 09.04.2021 07:20


Mathematics, 09.04.2021 07:20

Arts, 09.04.2021 07:20

Mathematics, 09.04.2021 07:20


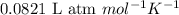
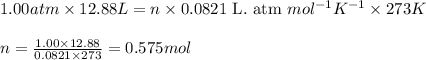
 .....(1)
.....(1)