

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
Do you know the correct answer?
NH4+(aq) + NO2−(aq) → N2(g) + 2H2O(l) is given by rate = k[NH4+][NO2−]. At a certain temperature, th...
Questions in other subjects:

Mathematics, 16.09.2019 06:00

History, 16.09.2019 06:00

Mathematics, 16.09.2019 06:00

Social Studies, 16.09.2019 06:00




History, 16.09.2019 06:00


Chemistry, 16.09.2019 06:00


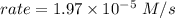
![rate = k[NH_4^+][NO_2^-]](/tpl/images/0540/2238/5a52f.png)
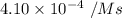
![[NH_4^+]=0.301\ M](/tpl/images/0540/2238/bd4cc.png)
![[NO_2^-]=0.160\ M](/tpl/images/0540/2238/7b7cf.png)
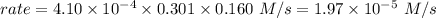
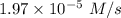 is the rate of the reaction at that temperature if [NH4+] = 0.301 M and [NO2−] = 0.160 M.
is the rate of the reaction at that temperature if [NH4+] = 0.301 M and [NO2−] = 0.160 M.



