
Chemistry, 10.03.2020 06:31, Halieyrobinson3003
Oxygen is supplied to a medical facility from a 30 ft3 compressed oxygen tank. Initially, the tank is at 2000 psia and 80°F. The oxygen is removed from the tank slowly enough that the temperature in the tank remains at 80°F. After two weeks, the pressure in the tank is 100 psia. Determine the mass of oxygen used in lbm. Also determine the total heat transfer to the tank in Btu. Treat the oxygen as an ideal gas with constant specific heats at 80°F.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
Do you know the correct answer?
Oxygen is supplied to a medical facility from a 30 ft3 compressed oxygen tank. Initially, the tank i...
Questions in other subjects:


Mathematics, 19.05.2021 19:40

Mathematics, 19.05.2021 19:40

Mathematics, 19.05.2021 19:40


English, 19.05.2021 19:40


Health, 19.05.2021 19:40

Mathematics, 19.05.2021 19:40

Mathematics, 19.05.2021 19:40


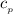 = 0.219 Btu/lbm.R
= 0.219 Btu/lbm.R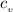 = 0.157 Btu/lbm.R
= 0.157 Btu/lbm.R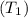 in the tank is given as 80°F.
in the tank is given as 80°F. in the tank from the ideal gas equation can be calculated as:
in the tank from the ideal gas equation can be calculated as: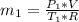
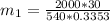
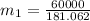
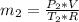
 = 540 R
= 540 R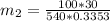
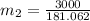
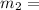 16.57 lbm
16.57 lbm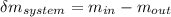
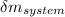 = change in the initial mass and final mass of the oxygen in the tank
= change in the initial mass and final mass of the oxygen in the tank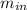 = the inlet mass of the oxygen
= the inlet mass of the oxygen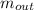 = the outlet mass of the oxygen
= the outlet mass of the oxygen
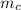 (i.e amount of oxygen used in the system)
(i.e amount of oxygen used in the system)
 -
-  =
= 
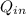 -
-  =
= 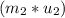 -
- 
 = specific enthalpy of the oxygen used
= specific enthalpy of the oxygen used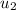 = specific internal energy of the final mass
= specific internal energy of the final mass = specific internal energy of the initial mass
= specific internal energy of the initial mass
 &
& 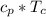
![[m_2*(c_v*T_2)]-[m_1*(c_v*T_1)]+[m_c*(c_p*T_c)]](/tpl/images/0540/2343/fb1ed.png)
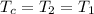
 = 16.57 lbm
= 16.57 lbm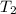 = 540 R
= 540 R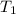 = 540 R
= 540 R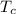 = 540 R
= 540 R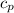 = 0.219 Btu/lbm.R
= 0.219 Btu/lbm.R = 0.157 Btu/lbm.R
= 0.157 Btu/lbm.R = [16.57×(0.157×540)]-[331.38×(0.157×540)]+[314.81×(0.219×540)]
= [16.57×(0.157×540)]-[331.38×(0.157×540)]+[314.81×(0.219×540)]



