Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations would be expected to neutralize. Assume complete neutralization.
a. A tablet containting 350 mg Al(OH)3 and 250 mg Mg(OH)2.
b. A tablet containing 970 mg of CaCO3.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 22:30, vhife4901
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
Do you know the correct answer?
Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations...
Questions in other subjects:



Mathematics, 31.07.2021 19:50


Mathematics, 31.07.2021 19:50



Mathematics, 31.07.2021 19:50



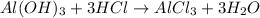
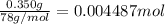
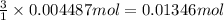 of HCl.
of HCl.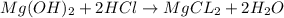
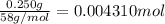
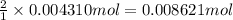 of HCl.
of HCl.
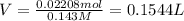
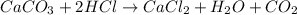
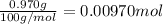
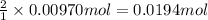 of HCl.
of HCl.