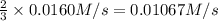Chemistry, 10.03.2020 04:41, flynwildozfuf5
Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of −0.0160 M/s. At what rate is ammonia being formed?

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
Do you know the correct answer?
Consider the reaction: N2(g) + 3H2(g) → 2NH3(g) Suppose that a particular moment during the reaction...
Questions in other subjects:

History, 30.06.2019 02:40


English, 30.06.2019 02:40

English, 30.06.2019 02:40


Mathematics, 30.06.2019 02:40

Mathematics, 30.06.2019 02:40




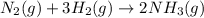
![R=\frac{-1}{1}\frac{d[N_2]}{dt}=\frac{-1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/a62ba.png)
![-\frac{d[H_2]}{dt}=0.0160M/s](/tpl/images/0539/9987/d5dae.png)
![-\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/527e4.png)
![\frac{-1}{3}\frac{d[H_2]}{dt}=\frac{1}{2}\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/2a2d1.png)
![\frac{-1}{3}\frac{d[H_2]}{dt}\times 2=\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/92027.png)
![\frac{1}{3}(-\frac{d[H_2]}{dt})\times 2=\frac{d[NH_3]}{dt}](/tpl/images/0539/9987/e9e1c.png)