
Chemistry, 10.03.2020 04:01, claudiasfandom
A piece of dry ice (solid CO2) weighing 17.0 g is placed in a 0.500−L bottle filled with air at 0.947 atm and 492.7°C. The bottle is capped, and the dry ice changes to gas. What is the final pressure inside the bottle

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Do you know the correct answer?
A piece of dry ice (solid CO2) weighing 17.0 g is placed in a 0.500−L bottle filled with air at 0.94...
Questions in other subjects:





Social Studies, 19.01.2022 20:00


Mathematics, 19.01.2022 20:00

Computers and Technology, 19.01.2022 20:00


Mathematics, 19.01.2022 20:00


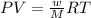
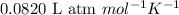
![492.7^oC=[492.7+273]K=765.7K](/tpl/images/0539/8543/60167.png)





