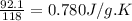Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
Do you know the correct answer?
The molar heat capacity of an unknown substance is 92.1 J/mol-K. If the unknown has a molar mass of...
Questions in other subjects:

English, 31.01.2020 06:58


Physics, 31.01.2020 06:58

Mathematics, 31.01.2020 06:58

Social Studies, 31.01.2020 06:58



Mathematics, 31.01.2020 06:58


Physics, 31.01.2020 06:58