Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
Do you know the correct answer?
Iodine-131 is a beta emitter used as a tracer in radio immunoassays biological systems. It follows f...
Questions in other subjects:

English, 08.09.2021 20:00

Mathematics, 08.09.2021 20:00

English, 08.09.2021 20:00


Mathematics, 08.09.2021 20:00


Mathematics, 08.09.2021 20:00




![[A_o]=8.0 g](/tpl/images/0539/8436/6a79c.png)
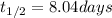
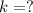
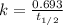
![[A]=[A_o]\times e^{-kt}](/tpl/images/0539/8436/abdec.png)
![[A]=8.0\times e^{-\frac{0.693}{t_{1/2}\times t}](/tpl/images/0539/8436/554b5.png)