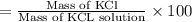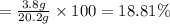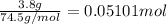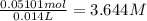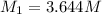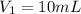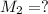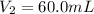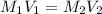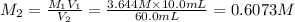Chemistry, 10.03.2020 03:19, jbrown76241
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and KCl solution is 44.30 g. After heating, the evaporating dish and dry KCl have a combined mass of 27.90 g.
(a) What is the mass percent (m/m) of the KCl solution?
(b) What is the molarity ( M) of the KCl solution?
(c) If water is added to 10.0 mL of the initial KCl solution to give a final volume of 60.0 mL, what is the molarity of the diluted KCl solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, jmanrules200
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 23:50, stephaniero6
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Do you know the correct answer?
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with...
Questions in other subjects:

Mathematics, 20.09.2020 15:01


Biology, 20.09.2020 15:01

Biology, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01