
Part A Which of the following statements about chemical formulas is FALSE? Which of the following statements about chemical formulas is FALSE? The subscripts represent the relative mass of each type of atom in the compound. Different compounds made of the same elements have different subscripts. The subscripts represent the relative number of each type of atom in the compound. The subscripts do not change for a given compound. All of the statements are true.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2


Chemistry, 23.06.2019 00:30, StayPuftMarshadowMan
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
Do you know the correct answer?
Part A Which of the following statements about chemical formulas is FALSE? Which of the following st...
Questions in other subjects:


Mathematics, 13.09.2019 09:10


Chemistry, 13.09.2019 09:10

History, 13.09.2019 09:10



Mathematics, 13.09.2019 09:10



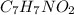 the subscripts present in it are 7, 7, 1, and 2.
the subscripts present in it are 7, 7, 1, and 2.



