Chemistry, 10.03.2020 02:57, MickeyAppleX
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2 Al ( s ) + 6 HCl ( aq ) ⟶ 2 AlCl 3 ( aq ) + 3 H 2 ( g ) What volume of H 2 ( g ) is produced when 2.80 g Al ( s ) reacts at STP?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
Do you know the correct answer?
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2 Al ( s ) + 6...
Questions in other subjects:


Mathematics, 19.07.2019 14:00



Mathematics, 19.07.2019 14:00

Social Studies, 19.07.2019 14:00

History, 19.07.2019 14:00

English, 19.07.2019 14:00

Mathematics, 19.07.2019 14:00

English, 19.07.2019 14:00


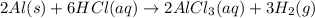
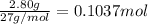
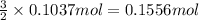 of hydrogen gas
of hydrogen gas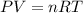 ( Ideal gas equation )
( Ideal gas equation )