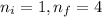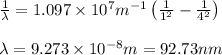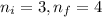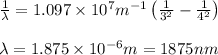Chemistry, 10.03.2020 02:57, urstruulyemily
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible region corresponding to the Balmer series in other series emmission lines are present in different regions of the eletromagnetic spectrum
Calculate the wavelenth of the n=4 to n=1 and the n=4 to n=3 transitions. Indicate in which regions of the electromagnetic spectrum these transitions would occur.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 01:00, deidaralove90
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3


Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
Do you know the correct answer?
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible...
Questions in other subjects:

Biology, 17.09.2019 02:30


History, 17.09.2019 02:30



Arts, 17.09.2019 02:30

English, 17.09.2019 02:30


Mathematics, 17.09.2019 02:30


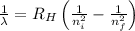
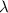 = Wavelength of radiation
= Wavelength of radiation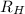 = Rydberg's Constant =
= Rydberg's Constant = 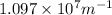
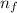 = Higher energy level
= Higher energy level 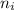 = Lower energy level
= Lower energy level