
Practice Problem 1: The following reagents are combined in a test tube: o 2.00 mL of 0.20 M potassium iodide o 1.00 mL of 1% starch o 0.50 mL of 0.20 M ammonium persulfate o 0.50 mL of 0.012 M sodium thiosulfate o 2.00 mL of 0.20 M potassium nitrate o 2.00 mL of 0.20 M ammonium sulfate What is the concentration of iodide in the solution in the test tube

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
Do you know the correct answer?
Practice Problem 1: The following reagents are combined in a test tube: o 2.00 mL of 0.20 M potassiu...
Questions in other subjects:

Health, 12.01.2021 06:00



Mathematics, 12.01.2021 06:00

World Languages, 12.01.2021 06:00

Social Studies, 12.01.2021 06:00

Mathematics, 12.01.2021 06:00


Social Studies, 12.01.2021 06:00

Chemistry, 12.01.2021 06:00


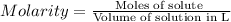
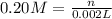
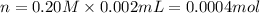
![[KI]=\frac{0.0004 mol}{0.008 L}=0.05 M](/tpl/images/0539/6847/fd685.png)
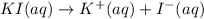
![[I^-]=[KI]=0.05 M](/tpl/images/0539/6847/8ce3a.png)




