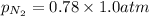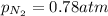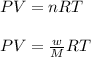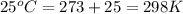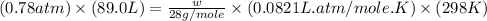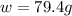Chemistry, 10.03.2020 01:16, andrew2786
Calculate the mass of nitrogen dissolved at room temperature in an 89.0 LL home aquarium. Assume a total pressure of 1.0 atmatm and a mole fraction for nitrogen of 0.78.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3


Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
Do you know the correct answer?
Calculate the mass of nitrogen dissolved at room temperature in an 89.0 LL home aquarium. Assume a t...
Questions in other subjects:

Mathematics, 01.02.2020 12:45

English, 01.02.2020 12:45


History, 01.02.2020 12:45

History, 01.02.2020 12:45


Mathematics, 01.02.2020 12:45




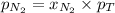
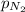 = partial vapor pressure of nitrogen = ?
= partial vapor pressure of nitrogen = ?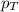 = total pressure = 1.0 atm
= total pressure = 1.0 atm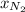 = mole fraction of nitrogen = 0.78
= mole fraction of nitrogen = 0.78